Which of the Following Molecules Has a Dipole Moment
Read More- NH3 intermolecular forces types Polarity and FAQ. Only for molecules with metallic bonds.
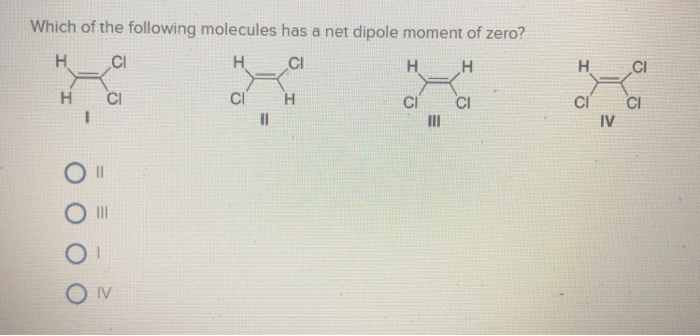
Solved Which Of The Following Molecules Has A Net Dipole Chegg Com
What determines their ionic strength.
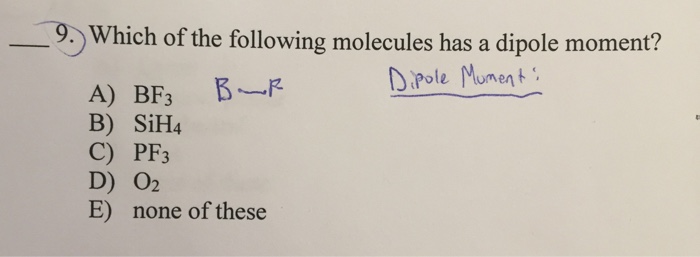
. B O2 c Cl2 d Br2 e l2 Which of the following has zero dipole moment ie. Dipole Moment D Propane CH3CH2CH3 44 01 Dimethylether CH3OCH3 46 13 Methylchloride CH3Cl 50 19 Acetaldehyde CH3CHO 44 27 Acetonitrile CH3CN 41 39 ACH3CN BCH3CH2CH3 CCH3OCH3 DCH3Cl ECH3CHO 1 2Of the following substances only _____ has London dispersion forces as its only intermolecular force. When the electron cloud of a molecule is easily distorted the molecule has a high _____.
Hydrogen bonding is a special case of _____. Only for molecules with polar bonds. Some molecules are nondipolar possessing no permanent moments.
A polarity B polarizability C dipole moment D van der Waals radius 17. Explain 1 SIZE distance of the atom size. Which of the following atoms should have the greatest polarizability.
And carbon-chlorine bonds are slightly stronger then carbon-hydrogen. Applies to charge and current distributions as well. PolarA molecule that has a dipole moment.
Increase with increasing the dipole moment Example. CH3OH NH3 H2S CH4 HCl ANH3 BH2S. Only for molecules with hydrogen bonding.
These forces are weak compared to the intramolecular forces such as the covalent or ionic bonds between atoms in a. Since CH3CN is much more polar its dipole-dipole forces are much stronger and its boiling and melting points Tb and Tm are much higher. Which of the following ionic compounds will be the least soluble in water.
A dipole-dipole forces B London dispersion forces C hydrogen bonding D covalent bonding 16. Bonds add vectorially to give a nonvanishing net dipole moment. This type of intraction is called dipole-dipole intraction.
CH3CH2CH3 and CH3CN have almost the same molecular weight and size. Only for molecules with nonpolar bonds. In the electric case the dipole moment is given by the product of one charge and the distance of separation.
Hydrogen Bonding IF between molecules containing H-atoms connected to highly electronegative. A H2O b NO21- c CCl4 d SO3 2- e HF Ionic bonds are the electrostatic attraction between oppositely charged atom. The moments of the two CO bonds cancel each other because of the rectilinear shape of the molecule resulting in a zero net dipole moment in the absence of electric field.
DipoleAny molecule that has both slight positive and negative charges on either end. PBF2 Ksp 33 x 10 8 PBF2 Ksp 33 x 10 8 A. Which of the following interactions are present between CO.
Intermolecular forces are the forces of attraction or repulsion which act between neighboring particles atoms molecules or ions. It mean it generate dipole moment between C-Cl and C-H. Which one of the following exhibits dipole-dipole attraction between molecules.
A London-dispersion forces B ion-dipole attraction C dipole-dipole attractions D ion-ion interactions E none of the above. Which of the following molecules has the shortest bond length. Opposite sign separated by a small distance.
The equilibrium between the soluble ions and insoluble salt can be described by the solubility. In the electric case a displacement of charge distribution produces a dipole moment as in a molecule. A XeF4 B AsH3 C CO2 D BCl3 E Cl2.
Therefore you can say that ch3cl has dipole dipole intraction. When NaCl dissolves in water aqueous Na and Cl- ions result. A common example is the CO2 molecule in Fig41b.
The distinguishing characteristic of the liquid crystalline state is the.
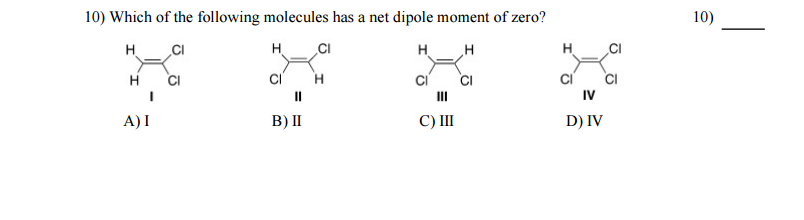
Solved Which Of The Following Molecules Has A Net Dipole Chegg Com
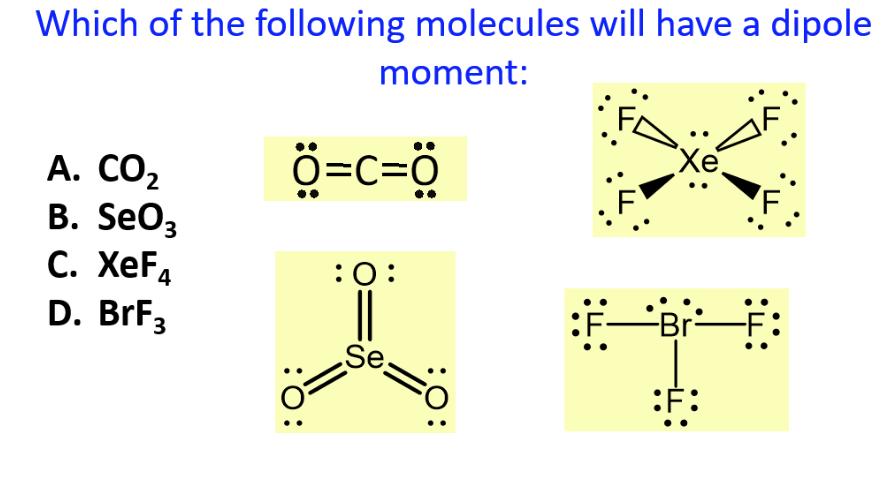
Solved Which Of The Following Molecules Will Have A Dipole Chegg Com
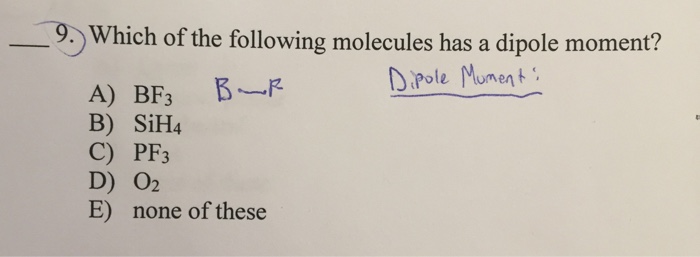
Solved Which Of The Following Molecules Has A Dipole Moment Chegg Com
Comments
Post a Comment